
Pharmaceutical labeling is a difficult topic to understand, much like the science behind the products themselves. There are myriad rules and regulations governing pharmaceutical labels, how they are applied, what information they offer, and more. Here we will break down some of the most common requirements so that you can be sure your labels stay in place, can be understood, and meet all legal obligations.
General Requirements for Drug Labels
The goal of pharmaceutical labeling is to inform consumers about the products they are using and keep them safe. To that end, pharmaceutical labels need to be informative and accurate, and they should present a simple summary for safe and effective use of the drugs inside. Furthermore, the label cannot be promotional, false or misleading, and should not make claims for uses that have not been tested and proven effective. Whenever possible, the data used must come from human trials and experiments.
Information Display Standards
In order to achieve the above goals, pharmaceutical companies must use a standardized format for presenting information so that consumers can easily identify and compare relevant information. This includes the official product name, active and inactive ingredients, the drug facts table, purpose and use, warnings, directions, and allergic reactions. Ideally, consumers can scan this table on the label and get an instant picture of whether the product is right for them or not.
Formatting Requirements
The FDA goes one step further with their requirements, noting that just having a table is not sufficient for informing consumers. Instead, they have additional requirements that must be met to keep formatting consistent across brands and product types. For instance, the font size and type must be large enough to be easily legible. The language must be verified to ensure that it is grammatically correct and that there are no implied benefits that have not been tested. Abbreviations may be used but must be common enough for the average reader to understand. There are also standards for the order in which each section appears in the table, how the information is displayed, and which facts are relevant to consumers. In some cases, the FDA also has guidelines for the use of special materials in packaging.
Quality Requirements
Once your label is printed, you are not out of the woods. There are also stringent requirements about the quality of label adhesion to ensure that labels do not fall off of your products after they leave your facility. All labels must be applied with a high level of consistency. The should be straight and free of wrinkles that could diminish the legibility of the data table. They should not peel away from the surface. In addition, most pharmaceutical products should have tamper-evident seals to protect the product inside. Naturally, these quality requirements should also be of the utmost importance to your company for the purposes of branding and marketing. You do not want to ship products out with faulty labels or without tamper-evident seals because it could cause consumers to doubt the effectiveness of your brand as a whole.
Choosing a Pharmaceutical Labeling Machine
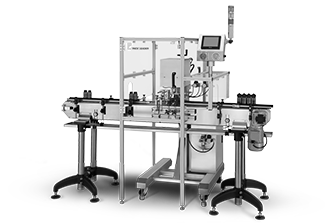
The real challenge of pharmaceutical labeling is trying to find a labeling machine that can meet the stringent requirements for your labels. Fortunately, the PL-521 is a perfect solution that was built specifically to handle a wide range of bottle and vial sizes, down to the smallest of pharmaceutical bottles. With one-button setup, the machine easily senses the size of your labels and allows you to fine-tune your label placement with manual positioning. Pack Leader's modular printer systems make a great addition to the PL-521, allowing you to add date and lot stamps to your products in a single pass. This machine offers state-of-the-art functionality that is versatile enough to work both in-line and in semi-automatic mode. By neatly holding products on their side as they move through the label head, the machine achieves perfect placement and adhesion without damaging the products or containers.
Pharmaceutical labeling is challenging, but the PL-521 offers stress-free label applications in no time at all. When you partner with Pack Leader USA, you can also take advantage of our free labeling consultation and our experts will help you find the right solution for your packaging needs.
.webp?width=200&height=135&name=2x-Packleader-logo-large%20(1).webp)













.webp?width=360&name=2x-color-logo%20(1).webp)